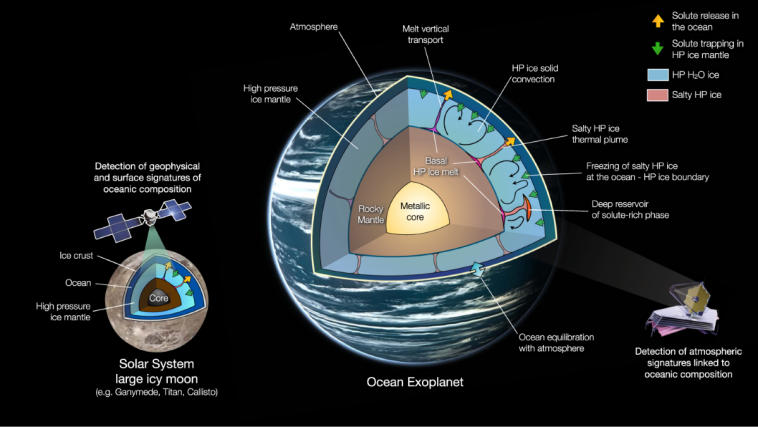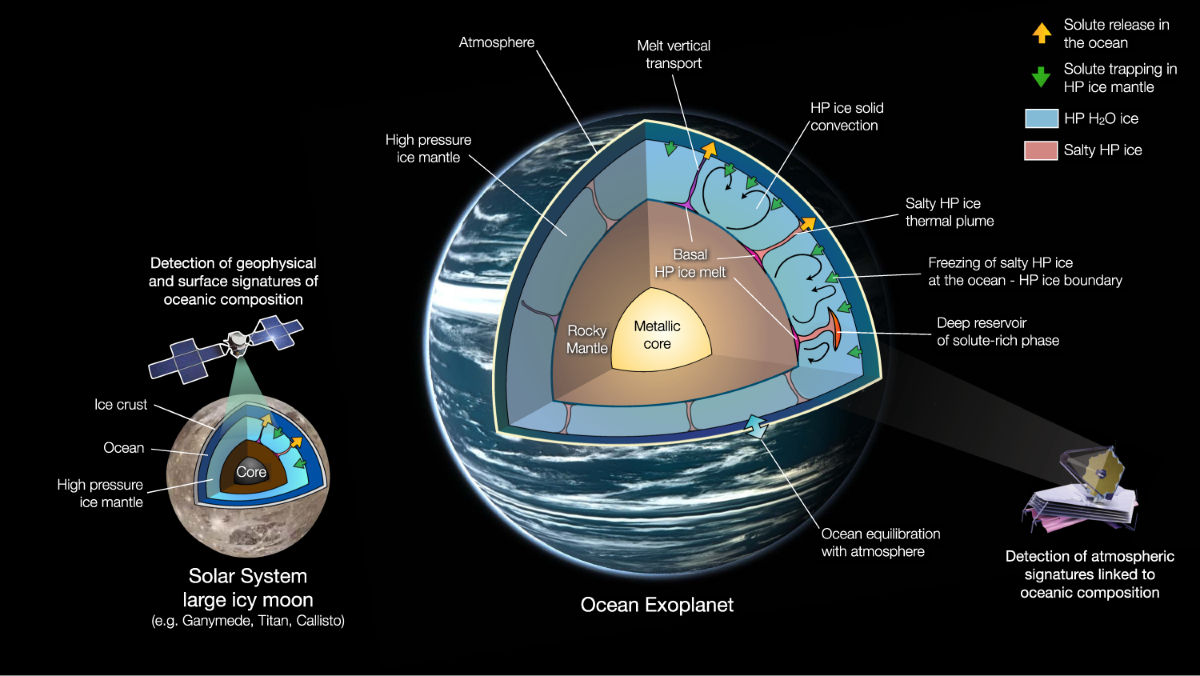
Exploring Alternative Liquids for Life on Waterless Worlds
The long-held view that water is the only liquid capable of supporting life on other planets is now facing a challenge. Recent laboratory experiments suggest that ionic liquids—salts that remain in liquid form even at elevated temperatures—might provide a hospitable environment for simple life forms on worlds where water scarcely exists. In this opinion editorial, we take a closer look at this fascinating research and discuss what it might mean for our search for life beyond Earth.
Traditionally, scientists have assumed that water—a substance essential for Earth’s biology—is a non-negotiable requirement for life elsewhere. But as we dig into new research findings, it becomes clear that the provision of a liquid medium, regardless of its composition, may be sufficient to jump-start basic metabolic processes. This idea pushes the boundaries of our understanding of habitability and opens up exciting, though challenging, opportunities for re-imagining where life might thrive in our universe.
Investigating the Formation of Ionic Liquids in Extraterrestrial Settings
For decades, the scientific community has been intrigued by the tricky parts of determining which planetary environments could foster the conditions necessary for life. The new research focuses on the formation of ionic liquids—fluids that have the unusual ability to stay liquid well beyond the boiling point of water. In controlled laboratory settings, a mix of sulfuric acid and specific nitrogen-containing organic compounds produced these liquids.
Considering that volcanic activity on rocky planets could produce sulfuric acid and that nitrogen-based organic compounds are frequently detected on asteroids and planetary bodies, the formation of ionic liquids might be a naturally occurring process far more common than previously thought. The experiments showed that these fluids remain stable at temperatures up to 180°C and under very low pressure conditions, scenarios that would be too harsh for conventional water-based chemistry.
Ionic Liquids: The Chemical Basis
At its core, an ionic liquid is a salt that stays in a liquid state under conditions that would typically vaporize water. The formation process, observed during experiments, involves a chemical reaction where sulfuric acid transfers hydrogen atoms to organic molecules, resulting in a complex fluid that defies the normal rules of evaporation. This discovery is built on years of meticulous research into chemical interactions under extreme conditions.
Key observations include:
- Stability at high temperatures and low pressures.
- Resistance to evaporation due to extremely low vapor pressure.
- The potential to support simple biomolecules, particularly certain proteins that remain stable in the ionic environment.
These features suggest that ionic liquids could create pockets of liquid on planets—even if these worlds are too warm or have too thin an atmosphere to maintain water in its liquid state.
Life Beyond Water: Reevaluating Habitability Zones
This groundbreaking work naturally leads to a broader discussion on the criteria that define a planet’s habitability. Traditionally, the search for life has been narrowly focused, assuming that water is indispensable. However, by broadening the definition, scientists are now considering the possibility of life based on alternative liquids. Such a perspective not only expands our view of where life might be found in our solar system, but it also encourages a reevaluation of exoplanet classification.
Rethinking habitability involves examining several key factors:
- Temperature Range: Water has a very narrow range of temperatures where it stays liquid. In contrast, ionic liquids can persist at much higher temperatures, broadening the scope of environments that might harbor life.
- Atmospheric Pressure: Planets with thin atmospheres that would normally lack water retention could still host ionic liquids that resist evaporation, even under such extreme conditions.
- Chemical Availability: The ingredients for forming ionic liquids, like sulfuric acid and nitrogen-containing organics, are common in the universe. This means that while water might be sparse, other chemicals might fill the gap.
When we take a closer look at these factors, it opens up a world of possibilities for where life could potentially exist. Worlds that were once dismissed as too harsh might offer alternative habitats in the form of liquid ionic reservoirs. This idea is both awe-inspiring and a little overwhelming, as it forces us to rethink the fine points of planetary science and astrobiology.
Implications for Future Planetary Exploration
NASA’s upcoming missions to Venus and other celestial bodies illustrate that space agencies are already ready to embrace unconventional ideas in the search for life. For instance, while Venus is notorious for its noxious sulfuric acid clouds, the very chemistry that makes its environment hostile might also create opportunities for discovering signs of life.
Here are some angles from which future exploration might benefit:
- Sample Collection and Analysis: Missions that collect atmospheric or surface samples need to consider that residual fluids might not evaporate completely. Instead, these fluids could be complex mixtures containing organic compounds, hinting at unexpected chemical processes.
- Instrument Design: The design of analytical instruments may need to account for the presence of ionic liquids rather than purely water-based solutions. This means rethinking methods for detecting life signals and biomarkers in such unconventional liquids.
- Data Interpretation: Researchers and engineers must be prepared to steer through subtle differences in chemical signatures. Recognizing whether these signatures come from water or other liquids could drastically alter our interpretations of data from distant worlds.
It is critical that space exploration programs adjust their strategies—a task as nerve-racking as it is exhilarating. Adapting to these insights involves not only rethinking the scientific instruments but also the theories behind planetary habitability. Only by integrating these new perspectives can we hope to unlock the full potential of the universe as a cradle for diverse forms of life.
Understanding Chemical Reactions in Extreme Conditions
One of the most compelling aspects of this research is how it brings to light the capabilities of chemical reactions under conditions that appear, at first glance, to be too extreme for life. The transformation of sulfuric acid and nitrogen-containing compounds into ionic liquids demonstrates that even simple chemical exchanges can lead to unexpected outcomes when subjected to high temperatures and low pressures.
This observation has broad implications beyond just astrobiology:
- Industrial Applications: On Earth, ionic liquids are primarily synthesized for industrial and technological uses. The insights from these experiments could lead to new methods for producing and applying these exotic chemicals in various high-tech and manufacturing processes.
- Material Science: The stability and unique properties of ionic liquids under extreme conditions might lead to innovative materials that can withstand high temperatures or low-pressure environments, which could be particularly beneficial in industrial settings.
- Chemical Engineering: Learning to work around the tricky parts of these chemical interactions offers a chance to develop engineered processes that can be applied both in space exploration and on our own planet.
By embracing these possibilities, industries and research institutions alike can figure a path to harnessing the potential of ionic liquids for applications ranging from energy production to advanced manufacturing techniques.
Considering the Broader Economic and Industrial Impact
While the primary focus of this research is astrobiology, the discovery of naturally forming ionic liquids could have broader implications for various economic sectors. From small businesses involved in cutting-edge research to large-scale industrial manufacturing, the ripple effects of these findings could prove to be extensive.
For example, industries might see:
- New Opportunities in Materials Science: The unique properties of ionic liquids could lead to the development of novel high-performance materials, driving innovation in automotive and electric vehicle manufacturing.
- Enhanced Production Methods: Better understanding the chemical processes could enable industries to optimize responses to extreme conditions, improving product durability and reducing production costs.
- Tax Incentives for R&D: As governments recognize the potential economic benefits of these discoveries, we may see changes in business tax laws designed to incentivize research and development in this promising area.
It is important for business leaders and policymakers to take a closer look at how these experimental outcomes could translate into industrial benefits. While the immediate implications might seem off-putting due to the underlying complexity of the chemical interactions, the long-term potential is super important for maintaining a competitive edge in global markets.
Reimagining Life As We Know It
The notion that life could thrive in liquids other than water might seem like a brain-teaser at first. However, if we poke around further, the concept offers a fresh perspective on the nature of life itself. Earth’s biosphere is deeply intertwined with water, but this does not necessarily mean that other forms of liquid cannot support alternate biochemical processes.
This radical shift in thought invites us to consider:
- Alternative Biochemistries: Life on other planets could operate on chemical principles that differ markedly from those on Earth. Ionic liquids might serve as the medium for metabolic processes, protein stability, and other essential functions.
- Adaptability of Life: Earth’s organisms have evolved to survive in some of the most intimidating environments imaginable. Similarly, extraterrestrial life forms may have evolved to harness the advantages provided by ionic liquids, even if those life forms are fundamentally different from terrestrial beings.
- Philosophical Implications: If life exists in forms based on alternative liquids, then our very definition of what it means to be “alive” must be revisited. This realization has the potential to reshape our understanding of biology, both on Earth and beyond.
By considering these possibilities, we begin to appreciate the little details that challenge our anthropocentric view of life. The new research encourages us to steer through the tangled issues of defining life in a broader, more inclusive sense—a perspective that is both invigorating and full of problems that demand creative, interdisciplinary solutions.
The Role of Planetary Geology in Supporting Ionic Liquids
Planets are complex systems where many factors intersect in unexpected ways. On rocky planets, the presence of basalt-rock surfaces can play a key role in the natural formation of ionic liquids. The porous nature of basalt allows the seepage of sulfuric acid into rock crevices, creating mini-environments that might just hold onto these exotic fluids for a surprisingly long time.
This interaction between geology and chemistry is a perfect example of how apparently disconnected processes can come together to create something remarkable. In the lab, researchers observed that even after the bulk of sulfuric acid evaporated, a consistent, stubborn layer of ionic liquid would remain. This discovery illustrates that planetary surfaces could harbor small oases where non-water-based life might sustain itself.
The potential processes driving the formation of ionic liquids on planetary surfaces include:
| Process | Details |
|---|---|
| Volcanic Outgassing | Releases sulfuric acid, a key reactant for ionic liquid formation. |
| Organic Compound Deposition | Occurs through asteroid impacts and space dust, supplying nitrogen-based material. |
| Rock-Fluid Interaction | Basalt surfaces allow fluids to seep into pores, stabilizing ionic liquid drops. |
| Evaporation Dynamics | Lower atmospheric pressures lead to rapid evaporation of sulfuric acid, yet leave behind the ionic liquid residue. |
Understanding these interactions not only helps in the search for extraterrestrial life but also provides new insights into the interplay between geology and chemistry across the universe. It opens up a host of intriguing questions, such as whether planetary histories marked by active volcanism might be more favorable to alternative biochemistries than previously assumed.
Unraveling the Nitty-Gritty of Chemical Reactions in Icy and Hot Planetary Climates
While the discussion often centers on extreme heat and low-pressure conditions, it is equally important to consider how ionic liquids might behave under cooler, albeit still non-water-rich, conditions. The stability of these fluids over a wide range of temperatures raises essential questions about how life might adapt to variable climates on exoplanets.
Several factors need to be taken into account:
- Temperature Variability: Unlike water, whose liquid range is extremely constrained, ionic liquids retain their state across a broad temperature spectrum. This provides a potential lifeline for planets that experience sharp temperature swings.
- Survival of Biomolecules: Experiments indicate that some proteins and other biomolecules can remain robust within ionic liquids. Future studies will need to find out which biomolecules not only survive but also thrive under these conditions.
- Chemical Energy Sources: Life requires an energy source to drive metabolism. In a medium like an ionic liquid, alternative energy sources—possibly derived from geothermal activity or chemical gradients—might be in play.
When we picture a planet where liquid ionic pockets serve as the only viable habitat, we are forced to grapple with the tiny twists of chemical and thermal dynamics that dictate life’s sustainability. Such environments are, in many respects, a delicate balance—a balance that is both precarious and filled with the promise of unlocking new forms of life.
Assessing the Potential for Ionic Liquid-Based Life Forms
One of the most stimulating aspects of this research is the possibility that ionic liquids may be capable of supporting life, even if that life looks nothing like what we see on Earth. The idea that life forms might adapt to, or even originate from, such unfamiliar conditions invites a paradigm shift in how we conduct biological research in extreme environments.
To assess this possibility, researchers are now considering:
- Biomolecular Stability: How do basic building blocks of life, such as proteins and nucleic acids, react when immersed in an ionic fluid? Preliminary evidence suggests that certain proteins are stable, which bodes well for the hypothesis.
- Metabolic Pathways: The traditional pathways that we associate with metabolism might not operate in ionic liquids. Alternative routes must be explored, using chemical reactions that fit the environmental conditions.
- Replication and Evolution: For any candidate life form, the ability to replicate and evolve is a super important marker. Will the chemical makeup of ionic liquids offer a stable environment for these processes to occur?
Critically, while ionic liquid-based life may be simple by Earth standards, it represents a complete rethinking of how biology might operate in a non-water world. It is a topic that is both inviting and intimidating, loaded with issues as scientists continue to work through the tangled issues of adapting life to wholly alien chemical environments.
Strategies for Future Research and Practical Applications
Looking ahead, several strategies emerge as researchers look to further validate these findings. From refining laboratory techniques to developing space mission protocols, scientists are gearing up to test the boundaries of our current models.
Key research strategies might include:
- Simulated Planetary Conditions: Expanding laboratory experiments to more closely mimic the varied and unusual conditions found on other planetary bodies will be essential. This might involve testing mixtures of sulfuric acid with different organic compounds across even more extreme pressures and temperatures.
- Robust Detection Techniques: Improving analytical methods so that instruments can effectively identify and analyze ionic liquids in situ is crucial. Innovation in spectroscopy and chromatography can provide the fine shades needed to distinguish between different liquid chemistries.
- Interdisciplinary Collaboration: Bringing together experts from fields as varied as industrial manufacturing, chemical engineering, and planetary sciences will help in addressing the little details that define the successful application of these discoveries.
In addition, small businesses and startups focusing on advanced chemistry and materials science may soon find themselves at the forefront of applying these new principles. With the appropriate investment in research and development, the industrial applications of ionic liquids could lead to the creation of novel products or more efficient production processes—making their economic impact as significant as their contributions to science.
In summary, the discovery of ionic liquids that may support life in waterless conditions is not just a revelation for scientists studying far-off planets. It is loaded with implications for multiple sectors here on Earth, from manufacturing to technology and even taxation policies related to R&D incentives. The opportunity to steer through these innovative twists and turns holds the promise of transforming how we both explore and utilize chemical phenomena.
Looking Beyond Earth: A Changing Perspective on Habitability
The idea that life might be sustained by liquids other than water calls for an overhaul of the rigid boundaries we have long drawn around habitability. It challenges not only our scientific understanding but also our imaginative capacity to create scenarios in which life can emerge and evolve in unexpected forms.
Some of the core considerations for this new perspective include:
- Diversity in Environmental Conditions: By acknowledging that a variety of liquids could serve as solvents for biological processes, we can expand the habitable zone far beyond the narrow strip where water exists.
- Reinterpreting Biomarkers: Conventional biomarkers, those small distinctions that indicate the presence of Earth-like life, may need to be redefined for environments dominated by ionic liquids. This requires significant rethinking, which might initially seem intimidating but is critical for future discoveries.
- Redefining Life’s Requirements: At a fundamental level, if metabolism in a liquid environment is what truly matters, then life’s building blocks might be far more adaptable than previously assumed. It invites us to poke around for evidence that life, even in its simplest forms, can thrive under conditions that we once deemed too extreme.
This reimagined framework for habitability is likely to influence future space missions, as well as how we interpret data from current satellite observations. It calls on researchers to take a closer look at planetary systems that once seemed barren and to reexamine the possibilities that might lie hidden beneath their rocky surfaces or within their thin atmospheres.
Economic and Policy Considerations in the Age of Ionic Liquid Research
Beyond purely scientific and industrial benefits, the implications of this research ripple out into economic and policy arenas. As governments increasingly recognize the super important role of scientific innovation in driving economic growth, policies may evolve to better support research in these uncharted areas.
From a policy perspective, there are a number of practical steps that could be taken:
- Tax Incentives: Governments might offer tax breaks or credits to companies and research groups who undertake high-risk, high-reward research into alternative fuels and materials based on ionic liquids.
- Public-Private Partnerships: Collaborative efforts between public research institutions and private companies could accelerate the development of new technologies capable of harnessing these unique chemical properties—potentially leading to groundbreaking applications in fields like aerospace, manufacturing, and even automotive technologies.
- Funding for Interdisciplinary Studies: Recognizing the tangled issues at the intersection of planetary science, chemistry, and industrial engineering, public agencies might increase funding for interdisciplinary research initiatives. These initiatives would encourage different academic and industrial groups to get into joint projects that explore the potential of non-water solvents in depth.
Such steps are not merely administrative; they represent a concerted effort to align economic policies with the realities of modern scientific discovery. As we broaden the search for life, rethinking not only which planets are habitable but also how research is funded and commercialized becomes key to ensuring that innovation continues to thrive.
Final Thoughts: Embracing the Unconventional in Science and Industry
The possibility that planets devoid of water could still support life through alternative liquids like ionic liquids is as revolutionary as it is provocative. It challenges our preconceived notions of what makes a planet livable and pushes us to figure a path forward that is inclusive of these unexpected chemical environments. The research discussed here is a reminder that scientific progress often comes from exploring the twists and turns of nature that defy our everyday expectations.
For business leaders, policymakers, and researchers alike, this discovery serves as a call to action. It is a chance to review and reconsider the established criteria for habitability, to reexamine industrial processes, and to reframe the economic benefits of embracing novel chemical environments. Far from being a niche scientific curiosity, the potential for ionic liquid-based life forms could have far-reaching impacts on how we understand both our place in the cosmos and the economic structures that support technological advancement.
As we look to the future, it is essential that we remain open to the idea that life—and the materials that sustain it—might manifest in forms we have only just begun to imagine. In this era of rapid technological and scientific advancement, every new discovery offers not just a glimpse into the far reaches of space but a new chapter in the ongoing narrative of human ingenuity. Whether it be exploring rocky exoplanets with thin atmospheres or creating new industrial applications based on exotic chemical reactions, the journey ahead is filled with promising, if sometimes intimidating, opportunities.
In conclusion, while water has long been hailed as the must-have ingredient for life, the emerging evidence behind ionic liquids challenges us to broaden our horizons. This is not just a win for astrobiology but a potential turning point for multiple industries that rely on innovative chemistry. By working through these confusing bits and complicated pieces of new research, we can prepare ourselves for a future where the search for life extends beyond the familiar and into the unknown realms of alternative biochemistry.
This research invites us to take a closer look, dig into the hidden complexities, and embrace a new era of exploration—one where the universe continues to surprise us with solutions that are as off-beat as they are scientifically intriguing. It is a reminder that the twists and turns of nature rarely conform to our expectations, and that sometimes, life finds a way even in the most unexpected liquid environments.
Ultimately, the discovery of ionic liquids capable of sustaining metabolic processes on waterless planets is a bold proposition. It challenges everything we thought we knew about habitability and requires us to figure a path through the maze of planetary chemistry with fresh eyes. As we continue to explore these research avenues, whether in laboratories or through space missions, the potential benefits—both scientific and economic—are bound to be as expansive as the universe itself.
Originally Post From https://news.mit.edu/2025/planets-without-water-could-still-produce-certain-liquids-0811
Read more about this topic at
Planets without water could still produce certain liquids …
The Habitable Zone